The science of cannabis extraction is built around the process of obtaining relatively pure concentrations of target chemical compounds, like THC and/or CBD, in a speedy and efficient manner. The resulting concentrate is useful for many applications, including pharmaceuticals and recreational edibles.
One popular approach for creating these compounds utilizes an alcohol (like ethanol, isopropanol, or methanol) to dissolve the oily cannabinoids and remove them from the plant material. The chemical used to dissolve the cannabinoids in this scenario is referred to it as a solvent and the end result is a solution, or mixture, of the solvent and cannabinoids. This article will look at the important step of solvent removal and recovery which involves separating the cannabinoids from the solvent.
There are several stages of purification required to produce an extract suitable for using in something like vape pens. Solvent removal is simply one of the earliest and most critical. It serves two important functions:
- It purifies the product, preparing it for possible further processing.
- If done properly, it collects the solvent so that it may be re-used, in effect, recycling it. This is called solvent recovery.
Our systems excel at removing and recovering alcohol solvents.
Solvent Recovery Basic Principles
To look at how solvent recovery works, it helps to understand how brewers make liquor or alcoholic spirits, because both of these processes utilize distillation. The distillation process involves separating components of a solution via boiling, taking advantage of the difference in boiling points between different compounds.
In a distillery setup, a single batch run through a still may only achieve a concentration 70-80% alcohol, but purities of 90% and higher can be achieved through multiple distillations. In our solvent recovery scenario, we still aim to evaporate and condense the alcohol, but we also save the cannabinoid residue that remains in the boiling chamber. The boiling points of different cannabinoids is much higher than the alcohol solvent.
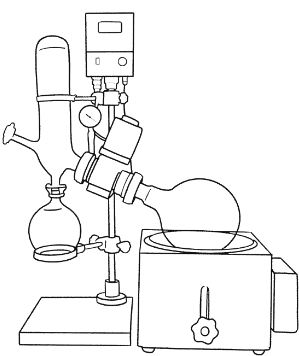
For solvent recovery, there are more specialized pieces of equipment that operate similarly to a still. One such device is the rotary evaporator, or “rotovap” for short. It has a bottom-heated chamber from which steam travels to another chamber for condensation, and then further on to collection. It also aids the movement of the vapor with suction provided by a vacuum pump and uses a cooled coil to increase the area available for the steam to condense on.
The boiling chamber is glass, and is suspended in a hot water bath, in which it rotates. This rotation allows for more of the liquid inside to distribute over the inner surface of the glass. This increases amount of solution in contact with the hot surface, which speeds up the evaporation and increases efficiency.
A More Effective Solution
Evaporation speed is directly related to the efficiency of heat transfer and that can be increased by enlarging the surface area or interface between the heat source and fluid. To visualize this better, let’s work through a few examples.
In the figure above we have two vessels, both with the same amount of room-temperature water inside them. If they are both turned on at the same time, which will start to boil faster? Vessel 1 has a wide surface in contact with heat source, while vessel 2 has a much narrower surface area in contact with the heat source. Therefore, even though the same amount of heat is being applied to the bottom of each vessel, the water in vessel 1 will boil faster, because more heat is transferred to the water.
Now let’s repeat the experiment, but instead of applying the heat from the bottom, we will apply heat from the sides. This time vessel 2 will boil first because it has more surface area exposed to the heat. In fact, the taller we make vessel 2, the greater it’s capacity to boil liquid.
For our final experiment, let’s replace the water in the vessels with a solution of alcohol solvent and product. Building on the results from the second experiment, we can also continue to increase the height of the vessel. The increased surface area increases the rate of boiling so much that the solution begins to boil before even reaching the bottom of the vessel. This means we can now remove the bottom of the vessel, turning it into a tube which can be continuously fed solution through the top. In addition, the cannabinoid residue can be separately collected from the bottom of the tube, while the alcohol steam is vented out to the side.
If you really wanted the best rate of recovery, you would align multiple such tubes in parallel, making efficient use of the available heating. At this point you would have a Falling Film Evaporator.
This setup has several advantages over the initial example with the basic vessels:
- Speed - Because the boiling chamber doesn’t simultaneously function as a collector of the cannabinoid residue, you don’t have to pause to collect residue from the chamber
-
Capacity - The only way to increase the capacity of a single rotovap is to use larger and larger glass balls. Eventually, that approach reaches a functional limit, where an operator can no longer handle such a large piece of glassware comfortably.
Some laboratories attempt to skirt around this by simply having multiple rotovaps, but eventually it is more efficient to have one machine that does the work of many.
Conclusions
The cannabis extraction industry is one in which alcohol solvents play a leading role. These solvents are a temporary addition used to dissolve cannabis oils and are later removed as part of the process of creating safe cannabis concentrates. Falling film evaporators represent a proven method of efficiently removing these solvents, and are a popular choice for industrial scale extraction.
Interested in buying an FFE? Contact us today for a quote on one of our FFE models.
Request a Quote